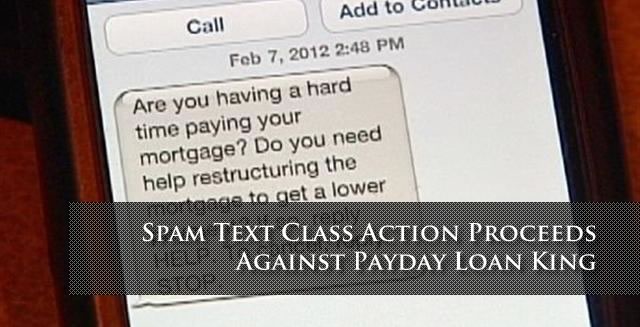
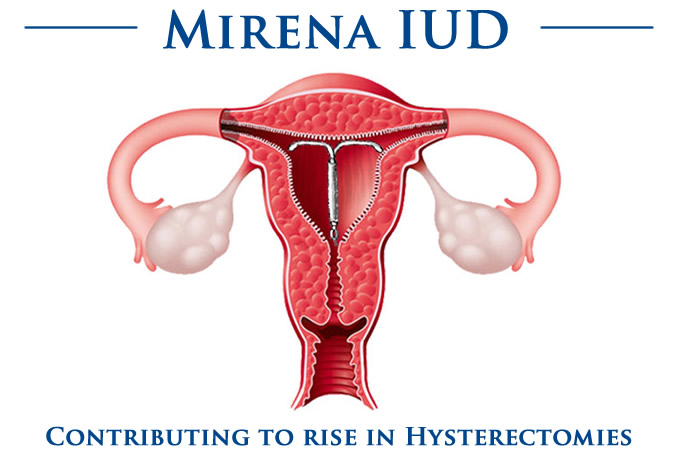
Neuriva Lawsuit Alleges False Advertising

Audet & Partners, LLP is investigating an increasing number of consumer complaints that have surfaced alleging false health benefits promoted by Schiff Vitamins, the manufacturer of Neuriva and Neurina Plus, two products included in a category of supplements commonly referred…